Mastering trial management
InSIGHT, our clinical trial management system (CTMS), is a powerful web-based, integrated information solution that consolidates, standardizes and visualizes operational and clinical data from multiple sources to generate a holistic view of all study information. InSIGHT allows you to turn your data into real knowledge, supporting better and faster decision-making throughout the life of a trial.
More than 25 years of experience distilled in our CTMS: INSIGHT.
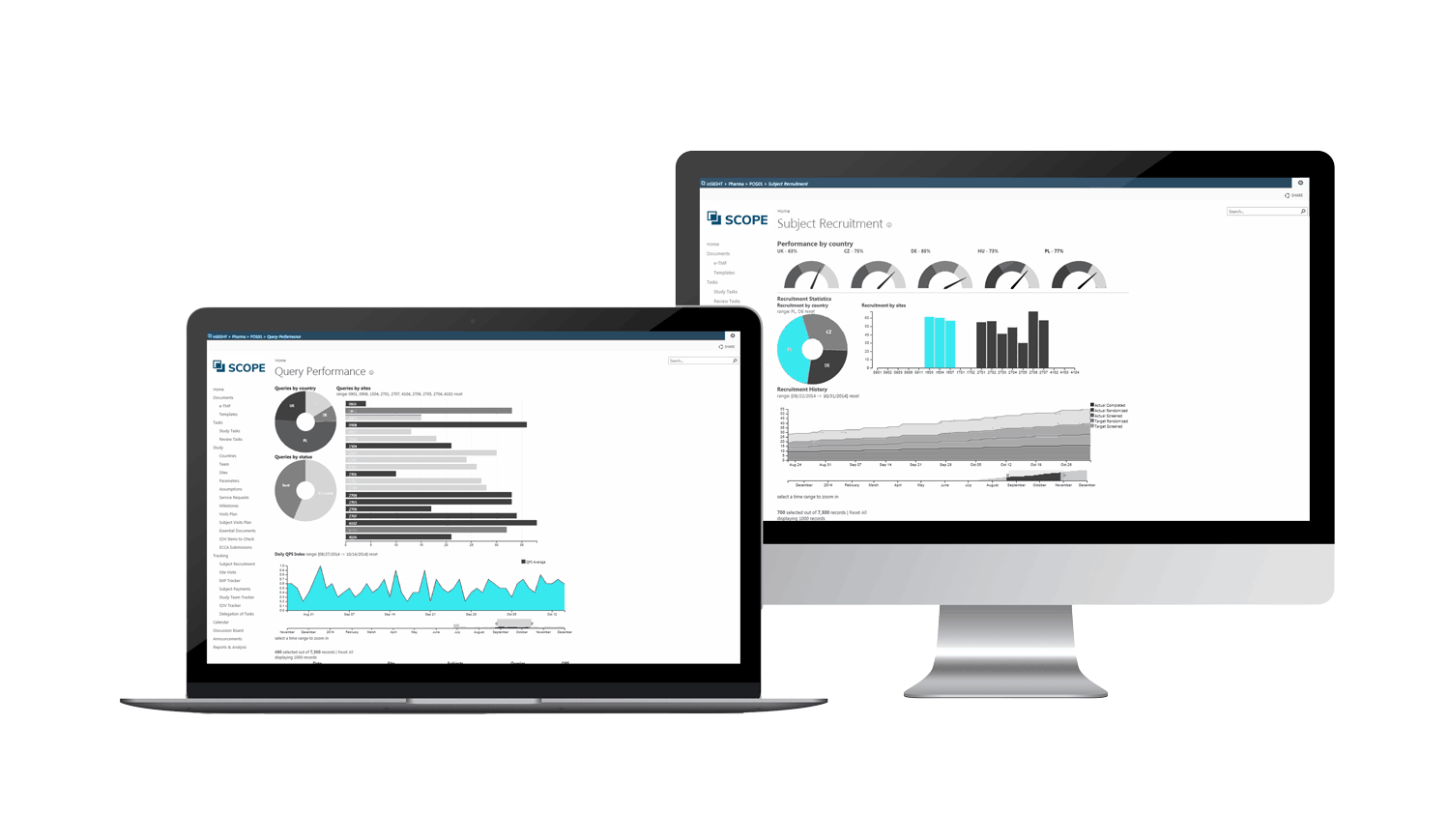
INSIGHT – AT A GLANCE
With InSIGHT, you have the tools and resources at hand required to manage and optimize your study:
COMPETENCE: InSIGHT’s development is based on operational excellence tailored to SCOPE’s processes.
CUSTOMER-FOCUS: Adjusted to the specific needs of clients and users, our custom CTMS provides the flexibility needed to integrate 3rd party systems driving a “Best-of-Breed” solution.
OVERSIGHT: Leave out the guess work and maintain a real-time overview with our sophisticated dashboards and trackers.
EFFICIENCY: InSIGHT’s powerful document management features support collaboration from initial draft stages to approval, e-signature and final release of the field document.
TRUST: Confidence and data integrity with controlled access to our integrated KaleidoSCOPE platform.

Our electronic signature
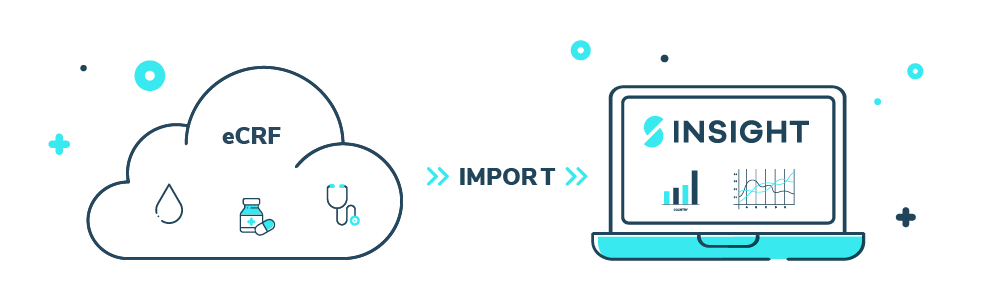
InSIGHT enables data import
PROJECT PLANNING
Set your project-specific expectations for essential documents, subject recruitment, monitoring visit (report) timelines and more
PROJECT TRACKING & VISUALIZATION
- From action items to insurances, protocol deviations, regulatory submissions, subject recruitment, site status and many other areas – InSIGHT keeps track of data points from A-Z
- Essential Documents Tracking and Monitoring Visit Report Documents Tracking are based on project specific document plans. Automated tracking occurs based on InSIGHT document workflows
- The Performance Dashboards allow for overview at the desired information level: study, country, site
- Built-in task management provides a transparent and efficient way of accomplishing all tasks
- Alerts can be flexibly set and used to stay informed on how the documentation progresses
- Overviews provide information about the involved study teams, countries and sites
DATA IMPORT
___Subject Data Import can be enabled regardless of which (e)CRF is being used
- Makes manual subject entry in InSIGHT obsolete
- Feeds into all required documents, trackers and dashboard
- Eliminates discrepancies between different data sources
DOCUMENT MANAGEMENT
- The Document Template Manager allows for study-specific adaptation of document templates
- InSIGHT populates documents automatically with system data
- Flexible Document Workflows can be assigned to individual system users or user groups
- Co-Authoring speeds up document finalization
- The PDF converter supports conversion to and combination of documents as pdf
- The E-signature feature allows for electronically signing of a document and verification of existing signatures
- The Document Filing Tool displays in your libraries which documents are already filed in your (e)TMF
___Further time-savers
- Batch Document Processing saves time
- Integration with MS Office Package allows for work with known applications
ELECTRONIC SIGNATURE
- Timely document signature loop completion and document authorization
- A verification tool can be optionally employed to ensure that the document content did not change since signature loop was started
CONFIGURATION & CUSTOMIZATION
- Role based access permissions for stakeholders of the specific project provide access on an ‘as needed basis’ and protection of confidential information
- Study Project Management grants user access to the study and / or individual Investigational Trial Sites based on operational needs
- Real-time access allows data analysis in accordance with the user’s areas of responsibility
COLLABORATION TOOLS
- InSIGHT´s collaboration tools facilitate and speed up shared efforts of the team
- Shared Document Repository allows for sharing of documents within SCOPE and with Sponsors
- Workflows & Task Notifications notify team members to relevant tasks
- Alerts / Reminders support planning of the daily working life
- Announcements keep the project team up to date with the latest project developments
- Custom MS Outlook-Add-in facilitates document sharing with non-InSIGHT users
GLOBAL CONTACT DATABASE
- SCOPE’s continuously growing internal contact database is far reaching and only accessible to SCOPE staff
- The database facilitates identification of suitable investigational sites for the trial in a timely manner
- Dedicated processes ensure that affected projects are notified of contact data updates that require follow-up action
CUSTOMIZATION & TECHNICAL SUPPORT
- Having the development team in-house allows us to swiftly and flexibly react to your additional project specific needs
- In-house user support is available for timely and straightforward issue resolution
QUALITY & REGULATORY
- InSIGHT is developed and validated per state-of-the-art approaches and globally accepted industry development standards, e.g. GAMP5
- InSIGHT is validated throughout its system life cycle to ensure that regulatory requirements such as ICH E6 and 21 CFR part 11 are met




